| 论文题目: | PcaO Positively Regulates pcaHG of the β-Ketoadipate Pathway in Corynebacterium glutamicum. |
|---|---|
| 作者: | Kexin Zhao, Yan Huang, Xi Chen, Nanxi Wang and ShuangJiang Liu* |
| 联系作者: | 刘双江 |
| 刊物名称: | Journal of Bacteriology |
| 期: | |
| 卷: | 192 |
| 页: | 1565-1572 |
| 年份: | 2010 |
| 影响因子: | 3.940 |
| 论文下载: | |
| 摘要: | We identified a new regulator, PcaO, which is involved in regulation of the protocatechuate (PCA) branch of the β-ketoadipate pathway in Corynebacterium glutamicum. PcaO is an atypical large ATP-binding LuxR family (LAL)-type regulator and does not have a Walker A motif. A mutant of C. glutamicum in which pcaO was disrupted (RES167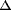 pcaO) was unable to grow on PCA, and growth on PCA was restored by complementation with pcaO. Both an enzymatic assay of PCA 3,4-dioxygenase activity (encoded by pcaHG) and transcriptional analysis of pcaHG by reverse transcription-PCR revealed that PcaO positively regulated pcaHG. A promoter-LacZ transcriptional fusion assay suggested that PcaO interacted with the sequence upstream of pcaHG. Electrophoretic mobility shift assay (EMSA) analysis indicated that an imperfect palindromic sequence (–78AACCCCTGACCTTCGGGGTT–59) that was located upstream of the –35 region of the pcaHG promoter was essential for PcaO regulation. DNase I footprinting showed that this imperfect palindrome was protected from DNase I digestion. Site-directed mutation and EMSA tests revealed that this palindrome sequence was essential for PcaO binding to the DNA fragment. In vitro EMSA results showed that ATP weakened the binding between PcaO and its target sequence but ADP strengthened this binding, while the effect of protocatechuate on PcaO binding was dependent on the protocatechuate concentration. pcaO) was unable to grow on PCA, and growth on PCA was restored by complementation with pcaO. Both an enzymatic assay of PCA 3,4-dioxygenase activity (encoded by pcaHG) and transcriptional analysis of pcaHG by reverse transcription-PCR revealed that PcaO positively regulated pcaHG. A promoter-LacZ transcriptional fusion assay suggested that PcaO interacted with the sequence upstream of pcaHG. Electrophoretic mobility shift assay (EMSA) analysis indicated that an imperfect palindromic sequence (–78AACCCCTGACCTTCGGGGTT–59) that was located upstream of the –35 region of the pcaHG promoter was essential for PcaO regulation. DNase I footprinting showed that this imperfect palindrome was protected from DNase I digestion. Site-directed mutation and EMSA tests revealed that this palindrome sequence was essential for PcaO binding to the DNA fragment. In vitro EMSA results showed that ATP weakened the binding between PcaO and its target sequence but ADP strengthened this binding, while the effect of protocatechuate on PcaO binding was dependent on the protocatechuate concentration. |

京公网安备 11010502044263号