The team led by Deng Tao and Gao Fu from the Institute of Microbiology has collaboratively uncovered the key mechanism by which the NS2 protein of influenza A virus drives the switch from viral RNA transcription to replication.
On January 29, 2025, a collaborative study by Researcher Deng Tao and Academician Gao Fu's team from the Institute of Microbiology, Chinese Academy of Sciences, was published online in Nucleic Acids Research. The paper, titled "Influenza A virus NS2 protein acts on vRNA-resident polymerase to drive the transcription to replication switch," reveals the dynamic regulatory mechanism by which the NS2 protein functions as a "switch" during viral infection, driving the transition of viral RNA from transcription to replication.
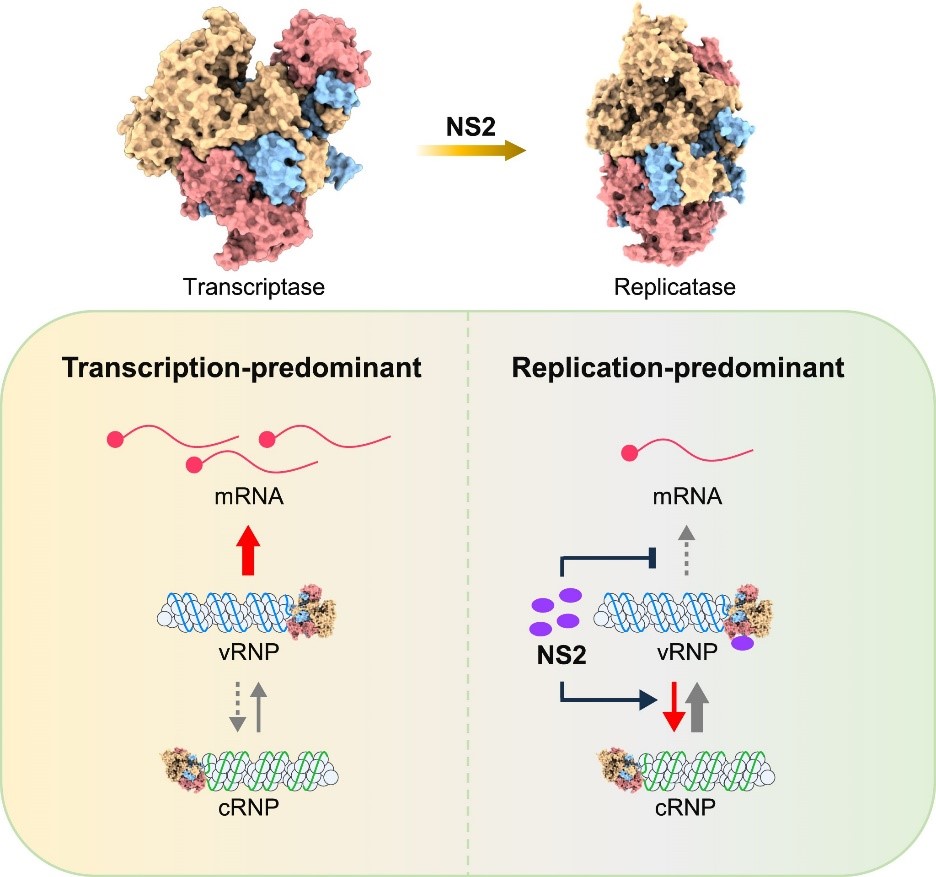
During the lifecycle of the influenza virus, the viral genomic RNA undergoes efficient transcription (vRNA→mRNA) in the early stages to synthesize viral proteins, while in the later stages, it primarily undergoes efficient replication (vRNA→cRNA→vRNA) to produce more progeny viral genomes. The mechanism behind this shift from early transcription dominance to late replication dominance has long remained unclear. Deng Tao's team previously reported for the first time that the early-expressed viral protein NS1 and the late-expressed protein NS2 are involved in dynamically regulating viral RNA transcription and replication. Specifically, the NS2 protein exhibits dual functions: suppressing transcription and promoting replication (Journal of Virology, 2023). Using a recombinant mutant virus infection system, the team further revealed that the NS2 protein promotes replication by synergistically interacting with the viral polymerase (RdRp) (Journal of Virology, 2024). Additionally, by resolving the high-resolution structure (~3.0 Å) of the NS2-RdRp complex, they uncovered the molecular mechanism by which NS2 inhibits transcription by interfering with the binding of RdRp to the host Pol II CTD (EMBO Reports, 2024).
Building on these findings, this study first utilized a unidirectional viral RNA replication system based on mutations in the viral RNA promoter sequence, demonstrating that the NS2 protein significantly promotes the first step of viral RNA replication, vRNA→cRNA synthesis. Through promoter element transplantation experiments of vRNA and cRNA, it was found that specific nucleotides in the RNA promoter are key determinants of RNA synthesis activity. Using the highly sensitive NanoBiT technology for in vivo interaction analysis, the team further discovered that NS2 specifically acts on the vRNA-bound viral RNA polymerase to exert its regulatory role in promoting viral RNA replication. Finally, in vitro viral RNA replication system experiments also confirmed that the NS2 protein primarily promotes the first step of viral RNA replication, vRNA→cRNA synthesis. These findings innovatively propose a new mechanism for the regulation of influenza virus replication, providing important insights for developing novel antiviral strategies targeting the viral RNA synthesis process.
Original link: https://doi.org/10.1093/nar/gkaf027
DOWNLOAD: