The team led by Bi Yuhai and Academician Gao Fu from the Institute of Microbiology has jointly uncovered the cross-species transmission mechanism and potential risks of the H2N2 influenza virus.
The H2N2 influenza virus, which caused the 1957 "Asian Flu" pandemic resulting in at least 2 million deaths, transitioned into a seasonal influenza virus post-pandemic. However, after 1968, the virus gradually disappeared from the human population. The mechanisms behind its cross-species transmission and pandemic potential have remained unclear until now. Recently, the team led by Bi Yuhai and Academician Gao Fu has identified a new molecular mechanism by which the H2N2 pandemic virus binds to human receptors, issuing a warning about the public health risks posed by the currently circulating H2N2 avian influenza virus in poultry. The related research findings were published online in Nature Communications on November 19, 2024.
The HA gene of the H2N2 pandemic virus originates from avian influenza viruses, with the HA protein primarily responsible for binding to host receptors. The research team identified a novel H2N2 avian influenza virus capable of binding to both human and avian receptors. They also discovered that the H2N2 virus from the early stages of the 1957 pandemic exhibited similar dual-receptor binding characteristics. However, as the virus spread, it gradually adapted to humans, shifting to primarily binding human receptors. This suggests a dynamic adaptation process of the H2N2 virus to the human population during its spread.
Further research revealed that single mutations in the HA protein, specifically R137M or N144E, caused the 1957 H2N2 pandemic virus to transition from dual-receptor binding (human and avian) to primarily binding human receptors. Additionally, the N144S mutation in the HA protein was identified as the key factor enabling the novel H2N2 avian influenza virus to bind human receptors (Figure 1). At the protein level, the combination of the N144S mutation with Q226L or Q226L-G228S mutations allowed the novel H2N2 avian influenza virus to predominantly bind human receptors. The study identified two new critical sites (137 and 144) on the HA protein that influence receptor affinity, revealing a new mechanism for the cross-species transmission of H2N2 influenza viruses. It also discovered and warned of mutation patterns that enable H2 influenza viruses to bind human receptors.
Moreover, the novel H2N2 avian influenza virus was found to not only infect human cells but also rapidly adapt to and spread among mammals, albeit to a limited extent. Additionally, the HA gene of currently circulating H2Ny avian influenza viruses has evolved into an independent branch and has undergone antigenic drift compared to the H2N2 pandemic virus. Although antibodies against the H2N2 pandemic virus exist in populations born before 1968, these antibodies show little to no cross-reactivity with the novel H2N2 and circulating H2Ny avian influenza viruses, indicating a lack of effective immune protection against H2Ny avian influenza viruses in the human population (Figure 2). In summary, these findings suggest that the currently circulating H2Ny avian influenza viruses in animals pose a certain risk to humans.
The co-first authors of the paper are Sun Ju, a joint Ph.D. student of the Chinese Academy of Sciences and Shanxi Agricultural University, Jia Mingjun, a master's student, and Zheng Tianyi, a joint Ph.D. student of the Chinese Academy of Sciences and Zhejiang University. The corresponding authors are Researcher Bi Yuhai and Academician Gao Fu from the Institute of Microbiology, and Professor Tian Wenxia from Shanxi Agricultural University. The research was supported by the National Science Fund for Distinguished Young Scholars and other projects.
Link to the paper: https://doi.org/10.1038/s41467-024-54374-z
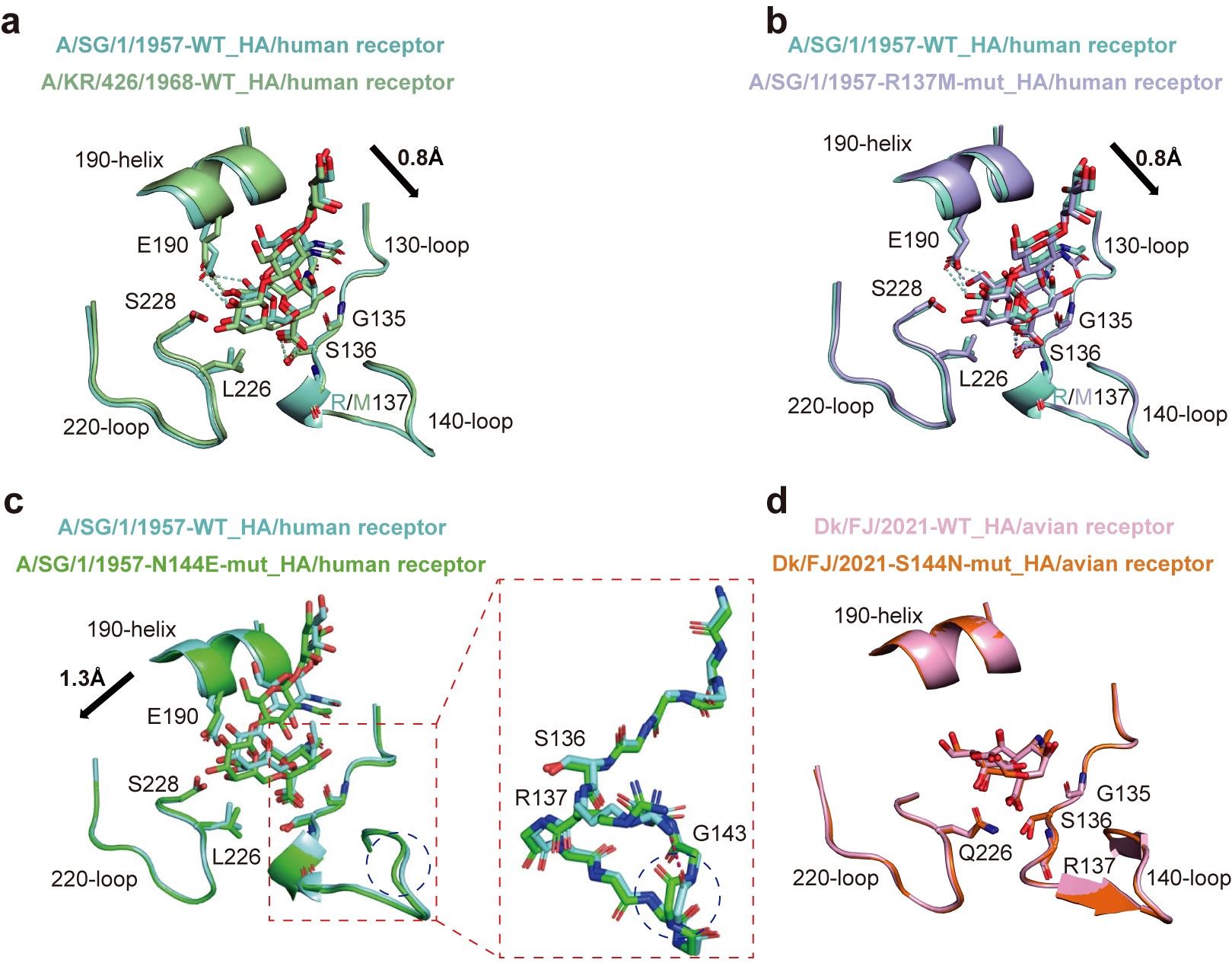
Fig. 1. Molecular mechanism by which amino acids at positions 137 and 144 affect the receptor binding properties of H2N2 influenza virus
a. Comparison of the structures of the H2N2 pandemic influenza A/SG/1/1957 and A/KR/426/1968 viral strains of HA protein-binding human receptor complexes; b. Comparison of A/SG/1/1957 wild-type HA and its R137M mutant-binding human receptor complex; c. Comparison of A/SG/1/1957 wild-type HA and its N144E mutant-binding human receptor complexes; d. Comparison of Dk/FJ/2021 wild-type HA and its S144N mutant binding avian-derived receptor complexes.
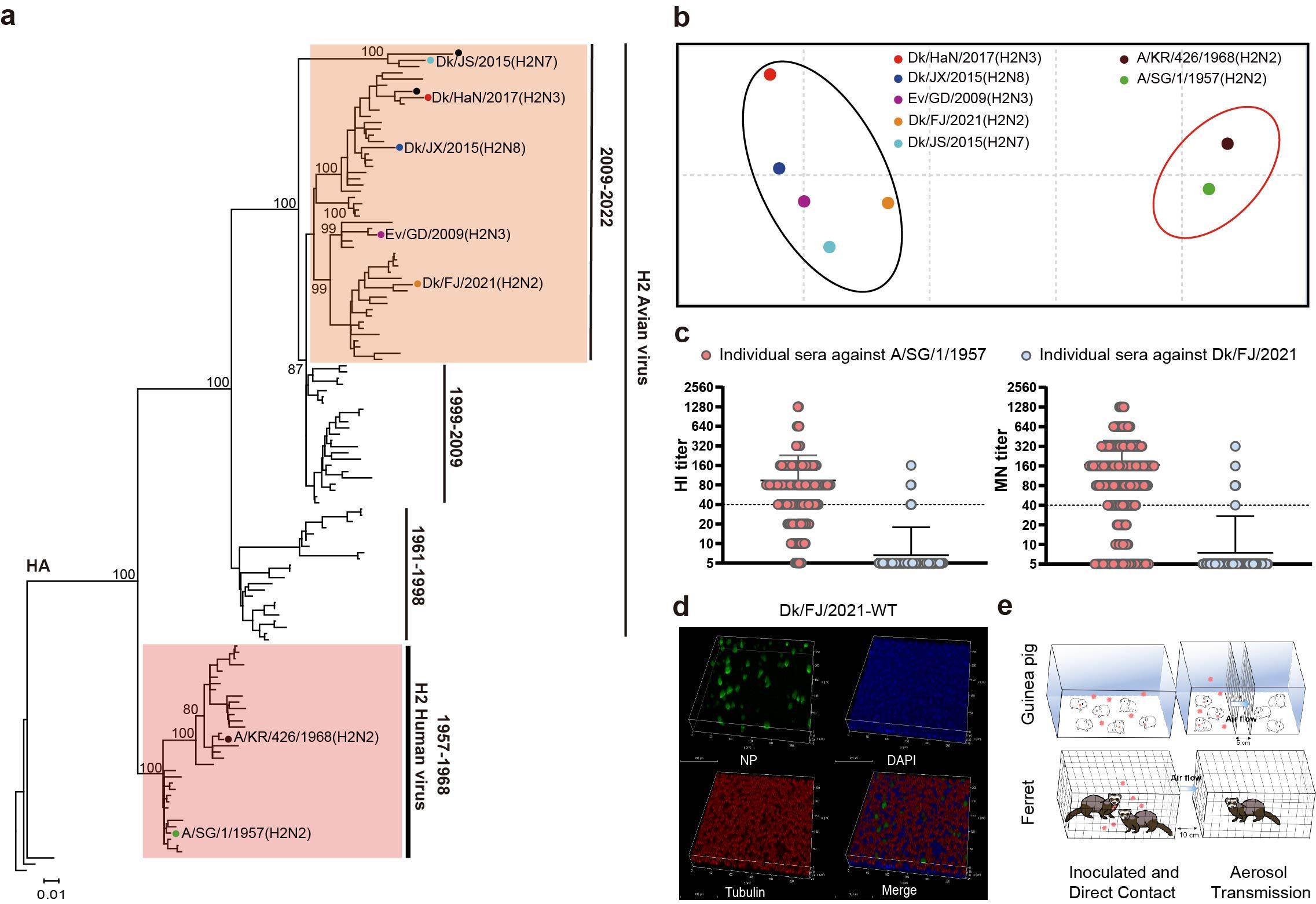
Figure 2: Currently circulating H2N2 avian influenza viruses pose a public health risk
a. phylogenetic tree of H2 influenza virus HA genes; b. antigenic profiles of H2 avian influenza and pandemic influenza viruses; c. serum antibody levels against H2N2 pandemic influenza and avian influenza viruses in populations born prior to 1968; d. H2N2 avian influenza infections in cells of human origin (NHBE); e. H2N2 avian influenza viruses are somewhat transmissible among mammals (guinea pigs and ferrets).
DOWNLOAD: