The team of Academician Gao Fu and Wang Qihui from the Institute of Microbiology researches and designs a novel monkeypox mRNA vaccine.
Recently, the research team led by Academician Gao Fu and Researcher Wang Qihui from the Institute of Microbiology, Chinese Academy of Sciences, published a paper in eBioMedicine titled "Single-chain A35R-M1R-B6R trivalent mRNA vaccines protect mice against both mpox virus and vaccinia virus." The team designed a series of chimeric trivalent mRNA vaccines targeting the mpox virus.
In July 2022 and August 2024, the mpox outbreak was declared a Public Health Emergency of International Concern (PHEIC) by the World Health Organization on two occasions. Currently, there are three attenuated live vaccines available globally for preventing mpox virus infection, but they still face issues such as insufficient immunogenicity and potential side effects from immunosuppressive genes. In recent years, the new generation of mpox vaccines has generally adopted the strategy of mixing multiple mRNAs encoding single antigens, which, while significantly reducing design complexity, has also increased the complexity and production costs of the vaccines, thereby reducing their accessibility.
Based on a structure-guided vaccine development platform, the team of Academician Gao Fu and Researcher Wang Qihui innovatively designed a chimeric trivalent mRNA vaccine using a strategy similar to that of the COVID-19 recombinant protein subunit vaccine. This vaccine can induce potent humoral and cellular immunity in mice, maintaining antibody levels nearly unchanged for almost six months post-immunization, and provided complete protection against a lethal dose of vaccinia virus challenge. It also significantly reduced the viral load in multiple organs of mice after mpox virus challenge. Under the same vaccinia virus dose, all mice vaccinated with the attenuated live vaccine of the vaccinia Tian Tan strain (VACV-VTT) died. As a chimeric mRNA vaccine, it encodes three mpox virus antigens in a single-chain mRNA, offering lower production difficulty compared to mixed vaccines, and thus is expected to have greater cost advantages, simpler production processes, and broader vaccine accessibility. Currently, the Institute of Microbiology has signed a technology licensing agreement with CSPC Pharmaceutical Group's JUSHI Biopharmaceutical Co., Ltd., authorizing them to further develop, clinically apply, and market the vaccine.
Kong Tianxiang, a joint Ph.D. student of the Chinese Academy of Sciences and Tsinghua University, Du Pei, an associate researcher at the Institute of Microbiology, Ma Renyi, a master's student, and Wang Han, an associate researcher at Peking University, are the co-first authors of the paper. Academician Gao Fu and Researcher Wang Qihui from the Institute of Microbiology are the co-corresponding authors. The research was funded by the National Natural Science Foundation of China and related projects of the Chinese Academy of Sciences.
Link to the paper: https://doi.org/10.1016/j.ebiom.2024.105392
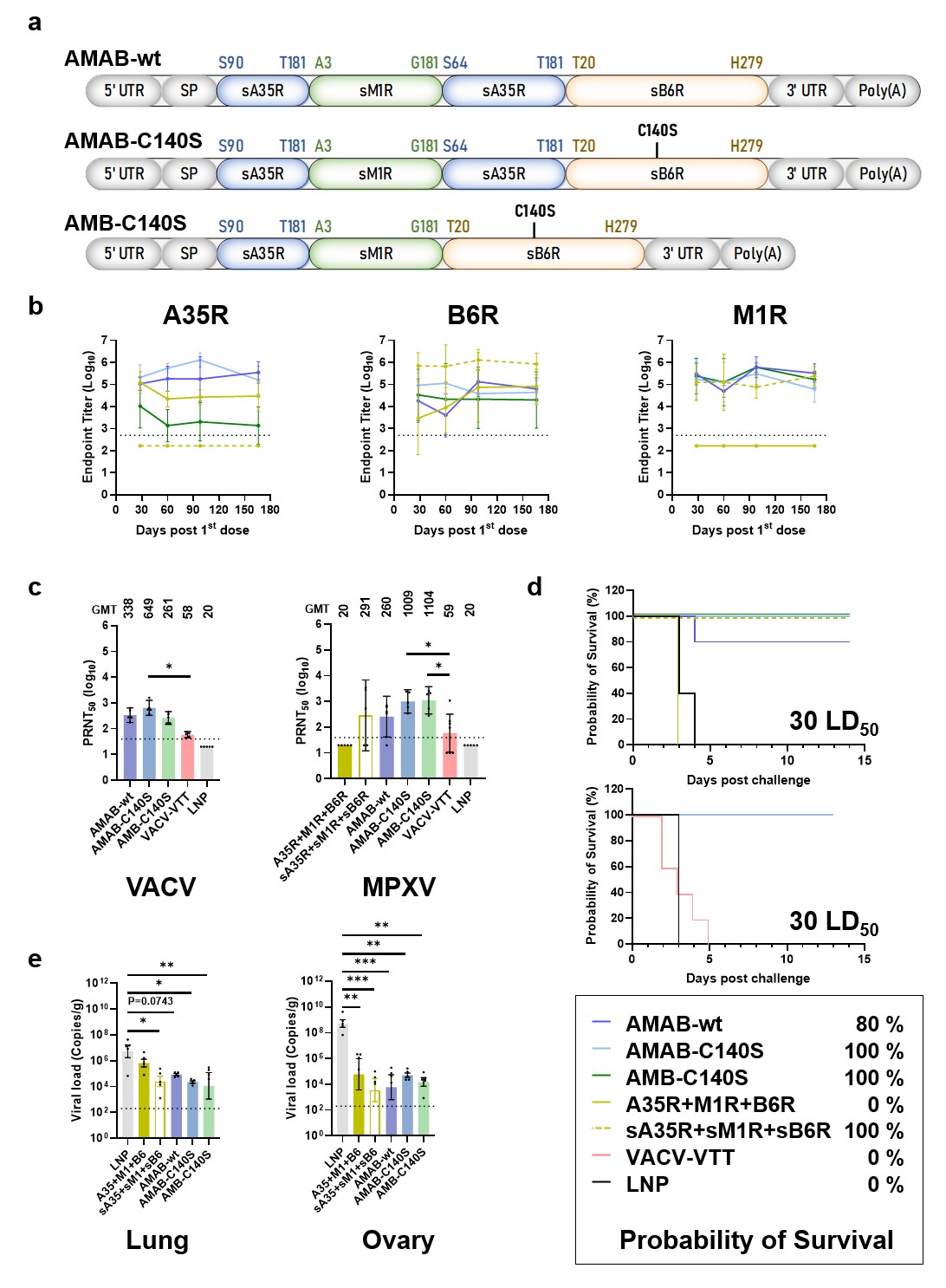
Figure 1: Design of the chimeric trivalent mpox mRNA vaccine (a), antibody titers and durability (b), neutralizing activity (c), protection against VACV challenge (d), and protection against MPXV challenge (e).
DOWNLOAD: