The team led by Academician Gao Fu at the Institute of Microbiology has uncovered the functional mechanism of the key replication protein component, type II topoisomerase, of the African swine fever virus and the mechanism of action of its inhibitors.
On August 21, 2024, Gao Fu's team at the Institute of Microbiology, Chinese Academy of Sciences, published a research paper online in Nucleic Acids Research entitled Structural basis for difunctional mechanism of m-AMSA against African swine fever virus The research paper is entitled Structural basis for difunctional mechanism of m-AMSA against African swine fever virus. The study analyzed the molecular mechanism of African swine fever virus (ASFV) type II DNA topoisomerase (Topo II) pP1192R-mediated alteration of DNA topology, revealed the inhibitor preference of pP1192R, the only known mammalian viral Topo II inhibitor, a novel inhibitor mechanism of action, and provided detailed information on drug targets. This study lays the foundation for the resolution of ASFV replication mechanism and provides new ideas for the development of antiviral drugs targeting the replication phase of ASFV.
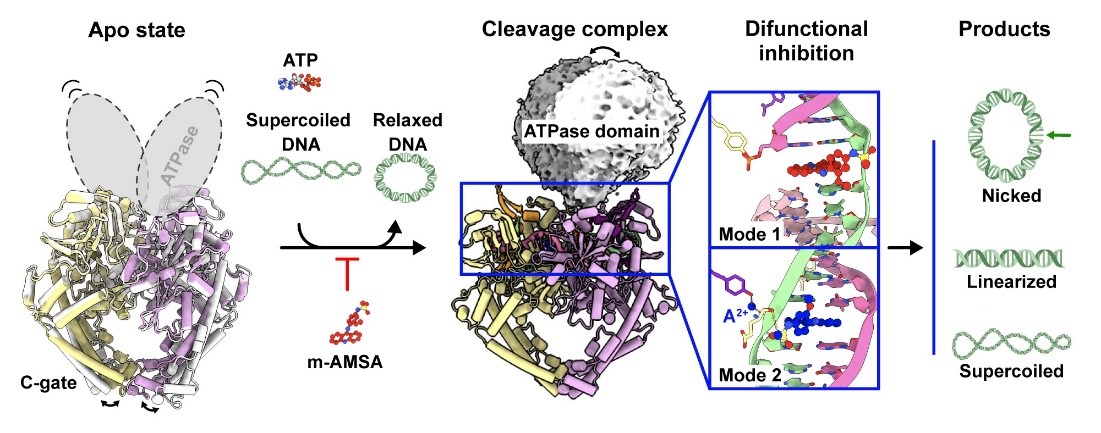
Figure 1: Graphical summary
DNA topoisomerases play key roles in core life processes such as DNA replication, transcription, recombination and repair, facilitating the precise conduct of these processes by introducing single-stranded or double-stranded breaks to unravel the superhelical structure of DNA. pP1192R, the type-II DNA topoisomerase of ASFV, is highly active in the mid- to late-stage of viral infection, and is a key enzyme in viral replication.
The team utilized a variety of structural biology tools to resolve the molecular conformation of pP1192R in multiple enzymatic phases of the full enzyme activity cycle. After comprehensively evaluating the targeting of Topo II inhibitors to pP1192R, it was found that only the eukaryotic Topo II inhibitor m-AMSA specifically blocked the enzymatic function of pP1192R and significantly inhibited ASFV replication in porcine alveolar macrophages (PAM). Subsequently, the research team resolved the structure of the pP1192R-DNA-m-AMSA ternary complex and revealed the dual inhibition mechanism of m-AMSA on pP1192R from both biochemical and structural perspectives. In addition to inhibiting the enzymatic function through the traditional mechanism of capturing the Topo II-DNA covalent complex, it was found for the first time that the Topo II inhibitor could inhibit the enzymatic function of Topo II through the mechanism of preventing DNA breakage and stabilizing the non-covalent Topo II-DNA complex. In addition, by resolving the conformation of pP1192R in the pre-cleaved state of DNA, the study identified for the first time the metal ion (A2+) bridging the gap between the Topo II DNA enzymatic active amino acid and the DNA phosphate backbone. This finding provides direct evidence for the dual ion-dependent DNA cleavage mechanism of Topo II.
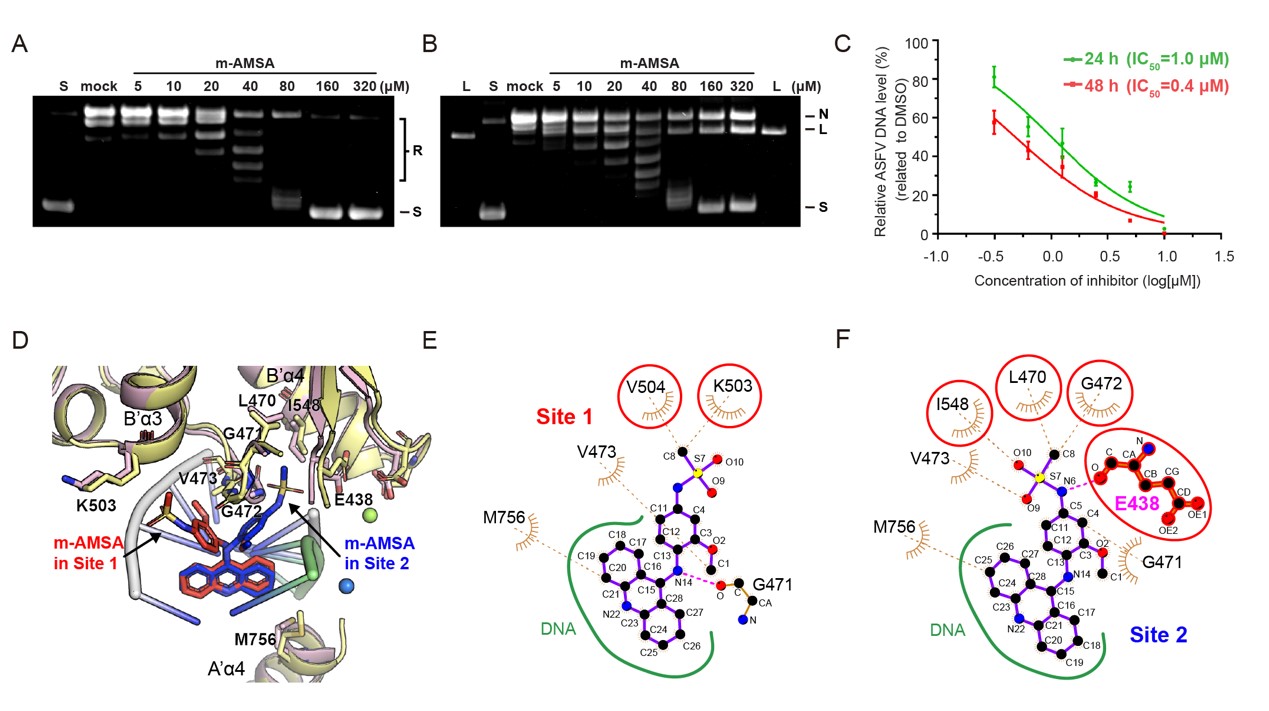
Figure 2. molecular mechanism of pP1192R inhibition by m-AMSA
Academician Gao Fu and researcher Qi Jianxun from Institute of Microbiology, Chinese Academy of Sciences, and associate researcher Wang Han from School of Future Technology, Peking University, were the co-corresponding authors of the paper. Ruili Liu and Yingxian Cheng, PhD students of Henan Agricultural University, Junqing Sun, PhD student of Shanxi Agricultural University, and Associate Researcher Lianfeng Li of Harbin Institute of Veterinary Medicine, Chinese Academy of Agricultural Sciences, were co-first authors of the paper. The study was financially supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China and the Discipline Construction Funds of Peking University.
Link to original article: https://doi.org/10.1093/nar/gkae703
DOWNLOAD: